From Hertz to Einstein: The Discovery of the Photoelectric Effect
The photoelectric effect was first observed by Heinrich Hertz in 1887 when he noticed that ultraviolet light caused sparks to jump more easily between metal electrodes. Later, Wilhelm Hallwachs and Philipp Lenard confirmed that light could eject electrons from metals. While classical wave theory could not explain these results, Max Planck’s idea of quantized energy in 1900 and Albert Einstein’s 1905 photon theory provided the breakthrough. Einstein proposed that light consists of particles, or photons, each carrying energy $E=hv$ . When this energy exceeds the work function of a metal, electrons are released instantly. This explanation, awarded the 1921 Nobel Prize, marked a turning point in modern physics.
Graphical Explanation of the Photoelectric Effect
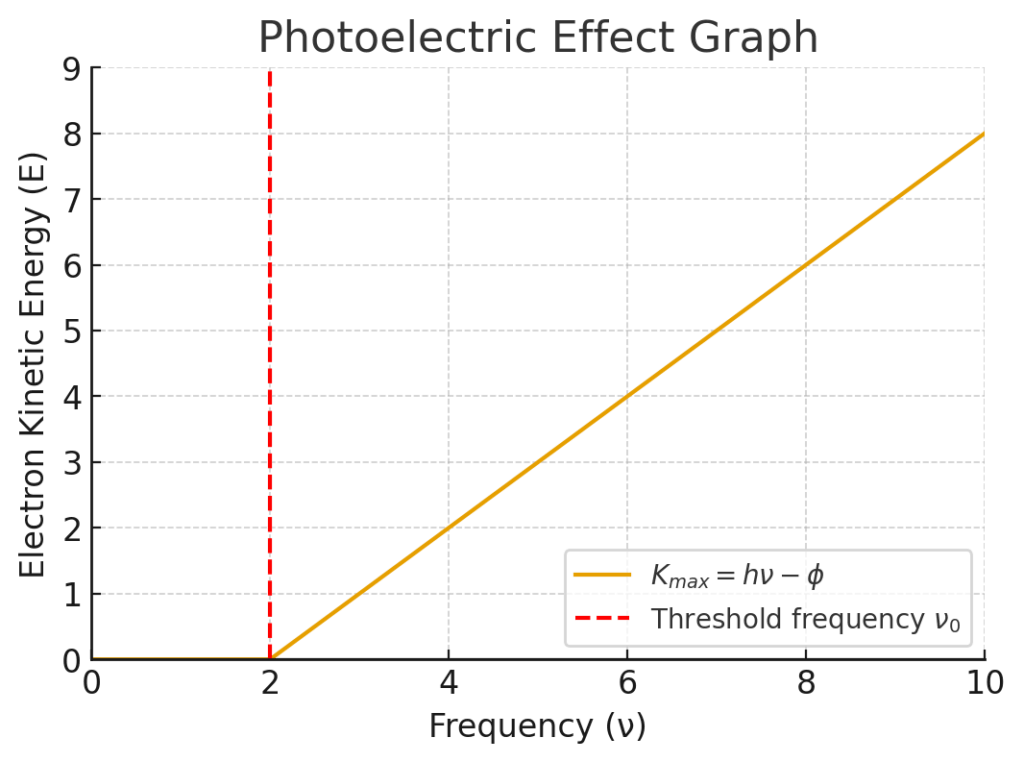
The photoelectric effect graph shows how electron kinetic energy depends on light frequency. Below the threshold frequency $v_0$, no electrons are emitted because the photon energy is less than the work function $phi$, the minimum energy needed to release an electron from the metal surface. Once $hv$ exceeds $phi$, electrons are ejected instantly with a kinetic energy that increases linearly as $K_max = hν−phi K_{max} $ This explains why light intensity alone cannot produce emission unless the frequency is high enough.
