Birth of Charles’s Law
In the late 18th century, French scientist Jacques Charles conducted experiments to investigate how the volume of gases changes with temperature. While flying hot air balloons, Charles observed that gases expand when heated. His careful measurements eventually led to the formulation of what we now call Charles’s Law, which describes the direct relationship between gas volume and temperature at constant pressure. Though his work wasn’t formally published at the time, it was later recognized and popularized by Joseph Louis Gay-Lussac.
Let’s focus on T, V
From the PV = nRT ideal gas equation, we can concentrate on the following four constants.
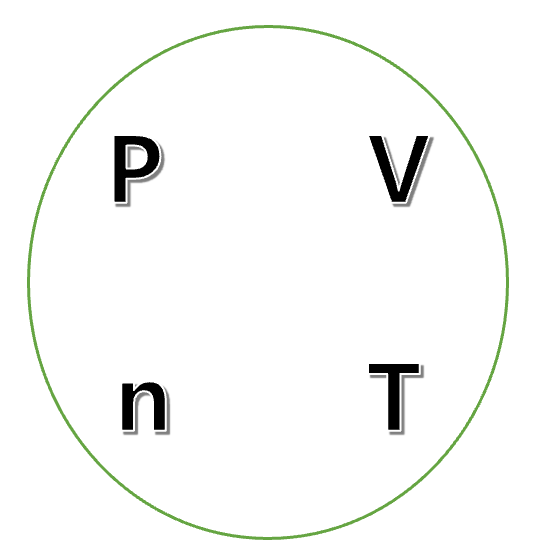
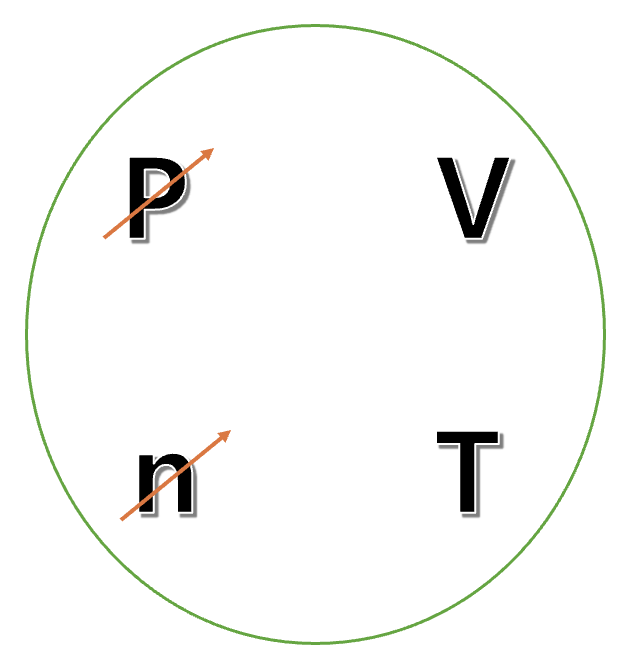
Since Charles’s law is expressed in the state where n and P are fixed, so we can focus on T and V.
$ PV = nRT $
Charles’s law holds when it is an ideal gas equation.
The main points in Charles’s Law are as follows.
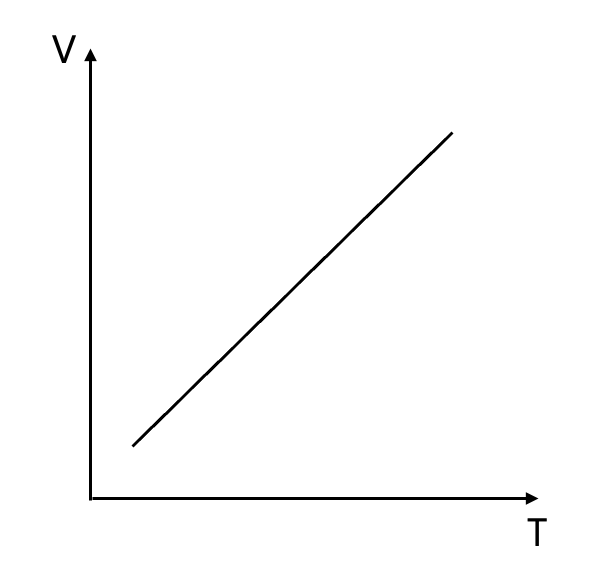
$ V \propto T $
$ \frac{V}{T} = k $
$ \frac{V_1}{T_1} = \frac{V_2}{T_2} $
$ \text{(T must be in Kelvin)} $
So if you put it as a graph, it’s like this.
Real-Life Applications of Charles’s Law
1. Basketball left outside in winter
Leave a fully inflated basketball outside on a cold day, and it will appear slightly deflated. As the temperature drops, the volume of the air inside the ball decreases, making it feel softer. This is why athletes often check air pressure before games — especially in outdoor sports.
2. Aerosol Cans Warning Labels
Most aerosol cans say “Do not store above 50°C” or “Keep away from heat.” When the gas inside is heated, it expands. Since the can is rigid, the pressure increases, and if the gas volume continues to expand with temperature (per Charles’s Law), it could lead to dangerous ruptures.
