Birth of Boyle’s law
In the mid-17th century, Irish scientist Robert Boyle conducted a series of experiments to investigate the behavior of gases. Working with Robert Hooke, he discovered a consistent relationship between the pressure and volume of a gas at constant temperature. In 1662, this observation was formally published, becoming what we now call Boyle’s Law — one of the earliest gas laws in chemistry.
Let’s focus on P, V
From the PV = nRT ideal gas equation, we can concentrate on the following four constants.
Since Boyle’s law is expressed in the state where n and T are fixed, so we can focus on P and V.
$ PV = nRT $
Boyle’s law holds when it is an ideal gas equation.
$ PV = \text{constant} $
At constant temperature, it is expressed as follows.
$ P \propto \frac{1}{V} $
$ PV = k $
The proportional representation is as follows.
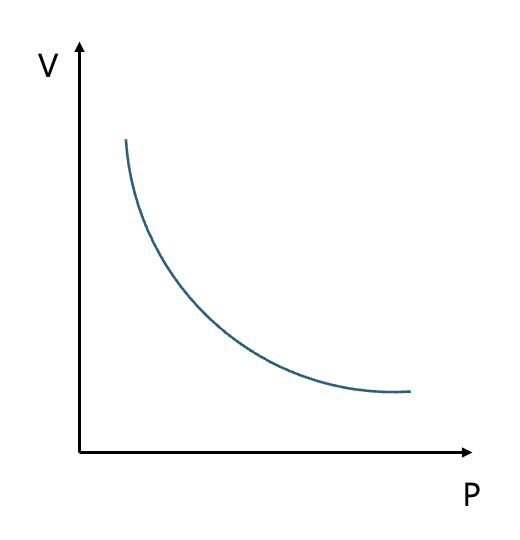
So if you put it as a graph, it’s like this.
Real-Life Applications of Boyle’s Law
1. Syringe
When you pull back the plunger of a syringe, the volume inside the barrel increases. According to Boyle’s Law, if the temperature remains constant, increasing the volume leads to a decrease in pressure. This lower pressure allows the surrounding fluid (like medicine or blood) to flow into the syringe.
→ As volume ↑, pressure ↓ → fluid is drawn in.
2. Scuba Diving
While diving, the pressure of the surrounding water increases as you go deeper. Boyle’s Law tells us that as pressure increases, the volume of gas in the diver’s lungs or air tank decreases. That’s why divers must equalize pressure and never hold their breath during ascent — the expanding gas volume can damage their lungs.
→ As pressure ↑, volume ↓
